Diseases
Coronaviruses in poultry
Chapter select
Control Tools
-
Diagnostics availability
-
Commercial diagnostic kits available worldwide
Diagnostic kits only available for infectious bronchitis virus (IBV). For IBV antibody detection, commercial ELISA kits are available worldwide. Hemagglutination antigens are available for some of the serotypes. Virus neutralisation tests are always in-house methods, availability is poor. Commercial PCR kits (especially genotype specific tests) are available in many countries. Many in-house PCR’s are used.
List of commercially available tests (Diagnostics for Animals)
GAPS :
- There are no properly validated guidelines for the interpretation of the titres of the commercial ELISAs.
- ELISAs (and other serological tests) are not able to discriminate between vaccine and field challenge derived antibodies.
- Sensitivity of the available PCRs is highly variable, false positive results are seen in proficiency testing schemes (PTS).
- Current genotype specific PCRs are not able to discriminate between vaccine and field strains of the same genotype. Interpretation of the Ct values is without guidelines.
- Sequencing makes differentiation between vaccines and field strains possible but this is hampered by the lack of validated markers for attenuation.
- Specific diagnostic kits required for other AvCoVs such as Turkey coronavirus (TCoV) and guinea fowl Coronavirus (GfCoV).
-
Commercial diagnostic kits available in Europe
See Section “Commercial diagnostic kits available worldwide”. List of commercially available tests (Diagnostics for Animals)
GAP :
See Section “Commercial diagnostic kits available worldwide”.
-
Diagnostic kits validated by International, European or National Standards
None
GAP :
No specific SOP’s or standards available. IBV is a moving target, new strains are detected every year, updating regularly is vital.
-
Diagnostic method(s) described by International, European or National standards
None
GAP :
No specific SOP’s or standards available. Many in-house PCR’s are used.
-
Commercial potential for diagnostic kits worldwide
Good. Many laboratories perform ELISAs for the detection of antibodies, the number of laboratories that perform PCRs is increasing.
-
DIVA tests required and/or available
No DIVA tests available. DIVA tests would be helpful to distinguish the live vaccines and the field strains of the same genotype. The same for the antibodies.
GAPS :
- Lack of genetic markers that are reliable to be used for DIVA.
- Vaccines (can) have quasi-species.
-
Opportunities for new developments
Tests that can reliably distinguish vaccines from field strains. Tests that indicate the level of cross-protection between the used vaccine(s) and the field strain.
GAPS :
- Lack of knowledge of the sites of attenuation of the available vaccines.
- Lack of knowledge about the basis of cross-protection, now mainly based on trial and error (performing vaccination-challenge studies).
-
Vaccines availability
-
Commercial vaccines availability (globally)
Vaccines are only available for IBV. No vaccines are available for other AvCoVs. Concerning IBV many live-attenuated vaccines (administered by spray or in drinking water) and inactivated vaccines (administered by injection) are available. The most widely used of the live attenuated vaccines is of the Massachusetts (see list below). DNA vaccines, sub-unit and peptide vaccines, virus-like particles, vector-based vaccines and reverse genetic vaccines have also been developed as proof of concept however, none have yet been commercialized.
GAPS :
- Lack of vaccines against other AvCoVs such as Turkey and Guinea fowl coronaviruses (limited number or absence of virus isolates).
- Better characterize AvCoV with enteric tropism.
- Better understanding of the persistence of vaccine strains in flocks.
- Identify a cell culture system that is permissible to infection with Enteric AvCoVs to facilitate vaccine development.
-
Commercial vaccines authorised in Europe
Many live attenuated and inactivated vaccines are available. Non-exhaustive list of vaccines available via this link.
-
Marker vaccines available worldwide
No
-
Marker vaccines authorised in Europe
No
-
Effectiveness of vaccines / Main shortcomings of current vaccines
Live attenuated vaccines: Most provide adequate protection against their homologous challenge virus; however, protection against heterologous challenge is variable. This can be improved by administering different live vaccines at the same time or in sequence with several weeks in between. This heavy use of live vaccines however could contribute to the continual genetic evolution of IBV.
Killed vaccines: Effective in boosting immune responses following vaccination with live attenuated vaccines but do not provide adequate protection alone. Method of delivery (intramuscular or subcutaneous injection) is complicated and time consuming.
Reverse genetic Vaccines : Are promising as proof of concept under experimental conditions, by nature have homogeneous viral genetic populations which should reduce viral genome evolution through selection. However are costly to produce and fall under GMO regulations.
GAPS :
- Lack of experimental evidence on whether the use of multiple live IBV vaccines are contributing the continual genetic evolution of IBV in the field.
- Need for an attenuated, highly immunogenic, broad protective IBV that can be rescued by reverse genetics in order to provide a good basis for vaccine design.
-
Commercial potential for vaccines
Many vaccines already in circulation and more will come (see Section “Commercial vaccines authorised in Europe”).
-
Regulatory and/or policy challenges to approval
No use of bivalent live attenuated IBV vaccines in France. Use of combinations of live vaccines is common in many areas of the world.
-
Commercial feasibility (e.g manufacturing)
Adequate.
-
Opportunity for barrier protection
In general, many farm workers shower in and out and wear farm specific clothing that stays at the farm and is washed there.
GAP :
This could be further improved: Gloves, face masks, temperature checks for workers are routinely done on many farms to prevent cross-species infection such as flu.
-
Opportunity for new developments
The list of references in this section is non-exhaustive.
Many different “molecular” vaccines have been developed and tested as proof of concept including
- DNA vaccines (Kapczynski, Hilt et al. 2003, Tang, Wang et al. 2007, Tian, Wang et al. 2008, Tan, Wang et al. 2009, Guo, Wang et al. 2010, Yan, Zhao et al. 2013),
- Virus-like particles (Chen, Huang et al. 2016)
- Vector-based vaccines (Johnson, Pooley et al. 2003, Falchieri, Lupini et al. 2013, Tan, Wen et al. 2019)
- Sub-unit and peptide vaccines (Yang, Wang et al. 2009, Cao, Wang et al. 2012, Cao, Wang et al. 2013)
- Reverse genetic vaccines (Cavanagh, Casais et al. 2007, Britton, Armesto et al. 2012)
The ability to specifically design the molecular composition of these vaccines means that they have high potential as DIVA vaccines.
GAPS :
Many of the published alternatives also have major disadvantages compared to the present commercially available vaccines.
This includes:
- application by injection
- Repeated vaccinations needed (expensive, not great for biosecurity as vaccination crews have to enter several times)
- Include only S1 of IBV
- Most likely less cross-protective (only S1).
-
Pharmaceutical availability
-
Current therapy (curative and preventive)
No curatives (antivirals) available.
GAPS :
- Antivirals that work for AvCoV’s?
- Only useful when very cheap, application by drinking water, feed or spray. Withdrawal period should be very short as well.
-
Future therapy
Very unlikely.
GAP :
Gammacoronavirus IBV and TCoV evolve quickly, would an antiviral treatment induce resistance to this drug easily?
-
Commercial potential for pharmaceuticals
Very low.
GAP :
Retailers and authorities should accept is before it could be used.
-
Regulatory and/or policy challenges to approval
Only useful when very inexpensive (few cents), application by drinking water, feed or spray. Withdrawal period should be very short as well.
GAP :
There are no antivirals available or allowed for food producing animals. New legislation would have to be developed.
-
Commercial feasibility (e.g manufacturing)
See section “Regulatory & policy challenges to approval”.
GAP :
Treatment should be very inexpensive (cents per chicken) to be attractive.
-
Opportunities for new developments
See section “Regulatory & policy challenges to approval”.
-
New developments for diagnostic tests
-
Requirements for diagnostics development
Molecular
Tools for genotyping AvCoVs should be based on the parameters proposed by the European COST action on avian coronaviruses. This means amplification and sequencing of the full S1 ORF (Valastro et al., 2016b).Tools that could distinguish vaccines and field strains of the same genotype.
Serological
It would be of interest in ELISA development to have strain specific tests.
GAPS :
- Lack of a reliable and cost-effective universal AvCoV molecular diagnostic tool for full S1 ORF amplification and sequencing.
- No ELISA tests capable of distinguishing the different AvCoVs. All are based on IBV. Earlier work based on the use of monoclonal antibodies (blocking ELISA) were not successful.
-
Time to develop new or improved diagnostics
Unknown, this would depend on the type of test developed.
-
Cost of developing new or improved diagnostics and their validation
Unknown, this would depend on the cost of the technology required and its availability.
-
Research requirements for new or improved diagnostics
Good communication between diagnostic laboratories and field veterinarians, so that any new tests meet demands.
Attempts should be made to obtain the complete genome sequences or as much genetic data as possible for the identified viruses.
Development of on-site tests to identify AvCoV infection would be very useful. However, these should be capable of distinguishing wild type virus from vaccine (certainly in broilers). Alternatively, positive on farm samples could be further submitted to a lab for sequencing to differentiate field from vaccine virus.
GAP :
Lack of complete genome sequence data for field and vaccine strains.
-
Technology to determine virus freedom in animals
RT-PCR tests are available and sensitive. Showing freedom of virus would require sampling every bird in a flock.
GAP :
Not realistic.
-
New developments for vaccines
-
Requirements for vaccines development / main characteristics for improved vaccines
Safe, highly effective, stable, inexpensive product that can be administered by mass-application unless a single vaccination in the hatchery would be highly effective and sufficient for protection), inducing high and long-lasting cross-protection against many different IBV strains, no or low interference with vaccines against other pathogens.
GAPS :
- What is the basis for cross-protection? There are many types of IBV, more will come. At the moment we do not know the basis of cross-protection. This hampers the development future vaccines that are more than just another attenuated strain (or inactivated antigen).
- It is unknown what level of protection is needed to prevent or minimise the risk of drift that might result in new types of IBV.
-
Time to develop new or improved vaccines
Traditional live attenuated vaccines usually take about 4-7 years to market. Inactivated IBV vaccines can be developed faster, however, these inactivated antigens are usually part of a multivalent vaccine (including other pathogens). Changing or adapting the IBV antigens in a vaccine means that all components have to be retested again: very costly and very time-consuming.
GMO vaccines (e.g. vector vaccines) for IBV are not yet on the market as the efficacy of these products has been moderate compared to the existing attenuated vaccines. Europe is also reluctant with accepting GMO vaccines compared to other parts of the world. Organic farming doesn’t accept GMO vaccines.
-
Cost of developing new or improved vaccines and their validation
Very costly, over 1 million euro (easily).
-
Research requirements for new or improved vaccines
Basic knowledge about the basis of cross-protection might make it possible to develop smartly designed vaccines that are more cross-protective than the existing vaccines.
GAP :
Fundamental knowledge is lacking.
-
New developments for pharmaceuticals
-
Requirements for pharmaceuticals development
Demands for using medicines in chickens are very high and increasing. Safety, withdrawal times, cost and application route (oral) are important considerations. See discussion in relation to antibiotic free production.
GAPS :
Even if there would be effective and very safe pharmaceuticals that can be applied at very low costs and in via a convenient application route, the demands of the customers (retailers, society, human health) go the other way.
IBV is mutating at a high rate, development of resistance is a risk with any new treatment.
-
Time to develop new or improved pharmaceuticals
Unknown, many years before it could be on the market.
-
Cost of developing new or improved pharmaceuticals and their validation
Unknown, very high.
-
Research requirements for new or improved pharmaceuticals
Unknown, however strict assessment of the potential for new pharmaceuticals to generate escape mutants should be a priority.
Disease details
-
Description and characteristics
-
Pathogen
There are currently three main coronaviruses (CoVs) affecting poultry industry IBV, TCoV and GfCoV, these are classified as follows according to the ICTV (2020):
Order: Nidovirales
Suborder Cornidovirineae
Family: Coronaviridae
Subfamily: Orthocoronavirinae
Genus: Gammacoronavirus
Subgenus Igacovirus
The family Coronaviridae within the order Nidovirales consists of two subfamilies: (1) Orthocoronavirinae comprising the genera Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus and (2) Letovirinae comprising the genera Alphaletovirus. Avian coronaviruses (AvCoVs) are of the genera gamma and delta. Those in poultry are almost uniquely of the genus Gammacoronavirus and those of small wild birds of the genus Deltacoronavirus. To date the principal AvCoVs are (See also Table below):
- Infectious bronchitis virus (IBV) first described in 1931 (Shalk & Hawn, 1931)
- Turkey coronavirus (TCoV) first described in the 1970s (Panigrahy et al., 1973; Ritchie et al., 1973)
- Guinea fowl coronavirus (GfCoV) first described in 2011 (Liais et al., 2014a)
These three viruses share a common genetic backbone but possess highly divergent genes encoding their surface glycoprotein (S) (Brown et al., 2016)
CoVs are enveloped and pleomorphic with an overall diameter of 60-120nm. Most CoVs contain four structural proteins: a large surface glycoprotein (spike or S protein visible as the corona), a small membrane protein (E), an integral membrane glycoprotein (M), and a nucleocapsid protein (N).
Table 1. Coronaviruses affecting poultry
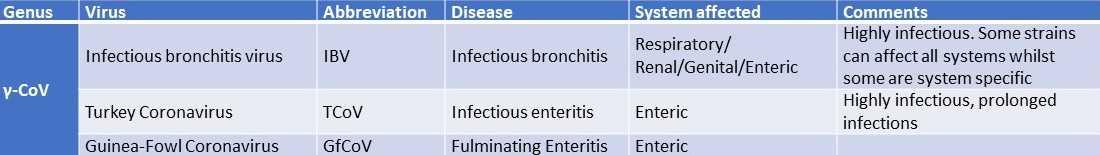
-
Variability of the disease
IBV: IBV’s have now been isolated in all parts of the world where chickens are farmed and exist as many different antigenic and genotypes (Sjaak de Wit et al., 2011; Valastro et al., 2016a). All have been isolated from chickens and most cause respiratory problems in this species however renal and genital diseases can also occur which can vary in severity according to the IBV strain involved. Severity of respiratory disease is usually higher in chickens bread for consumption (broilers) than in those used for reproduction or egg production (layers). In layers a drop in egg production can occur and this is usually more notable in older birds.Some isolates of IBV have a particular tropism for the kidney and are often associated with higher rates of mortality, especially at young age. Several IBV strains have been shown to replicate in the enteric tract however, the clinical relevance of this seems to be limited.
TCoV: In comparison with IBV there are very few TCoV isolates available for characterization. One virus has been isolated in Europe (France) and the rest have been isolated in the United states of America. These viruses were isolated from the intestines of turkeys with multifactorial enteric disease.Isolates from the USA have an IBV backbone of US origin with an S gene of unknown origin while the European isolate has an IBV backbone of European origin and also an S gene of unknown origin.
GfCoV: In line with TCoV there are a limited number of GfCoV isolates compared to IBV. These have all been isolated from the intestines of guinea fowl with fulminating enteritis. Disease in this species, as the name suggests, is almost always associated with mortality.Isolates share the same IBV backbone as the European TCoV with an S gene of unknown origin closely related to that of North American TCoVs.
GAPS :
IBV:
- Molecular basis of tissue tropism unknown.
- Why are respiratory problems in layers so variable?
- Varying persistence in repro and layers.
TCoV:
- The Role in multifactorial enteritis (poult enteritis complex and poult enteritis and mortality syndrome) in not fully understood.
- Persistence?
- Prevalence not known.
GfCoV:
- The role in fulminating enteritis not fully understood.
- Prevalence not known.
-
Stability of the agent/pathogen in the environment
As with other viruses of the family Coronaviridae: AvCoVs have been shown to survive for up to 10 days at an ambient temperature of approximately 20°C (Guionie et al., 2013) and for up to 20 days at lower temperatures (+4°C)
Most common disinfectants used in the poultry house inactivate AvCoVs. A broad overview of the effect of biocidels on CoV’s is listed in a review by (Kampf et al., 2020a, 2020b). CoV is more stable at a low pH than at a high pH. Survival times are increased when in litter containing faeces (Jackwood & de Wit, 2020).
-
Species involved
-
Animal infected/carrier/disease
Principally:
- Avian gammacoronavirus: Poultry
- Avian Delatacoronaviruses: Wild birds
-
Human infected/disease
None reported to date.
-
Vector cyclical/non-cyclical
None reported.
-
Reservoir (animal, environment)
Chickens are the natural reservoir for IBV.Turkeys for TCoV and Guinea fowl for GfCoV.IBV-like AvCoV’s (often just by PCR) have been detected in pheasants, peafowl, turkeys, teal, geese, pigeons, ducks and some other wild bird species (Cavanagh, 2005)Studies have shown that chickens can be susceptible for TCoV (Gomes et al., 2010).
GAPS :
- By PCR and sequencing of a limited part of the genome, avian coronaviruses like IBV have been detected in ‘unusual’ bird species, quite often in the surroundings of infected chickens. Very often, virus isolation was not attempted, antibodies against IBV were not tested, postulates of Koch were not tested and it remains unproven whether the birds were infected or just carried some RNA of IBV (e.g. inhalation of IBV positive dust).
- Several strains of IBV (like D1466, DE072, TC07-02) are very different from any known IBV strain and might be examples of interspecies transmission instead of the usual drift by mutations or recombination of the genomic material of known strains. So far, this is speculation.
-
Description of infection & disease in natural hosts
-
Transmissibility
IBV is very infectious, transmission parameter (R0) is 20 (De Wit et al, 1998). Transmission can be by aerosol, faeces and fomites (mechanical spreading).
TCoV is also extremely infectious with one infected turkey infecting another every 2.5h under experimental conditions. The virus is also infectious beyond the limits of detection by real-time PCR (Brown et al., 2018) and is excreted as infectious virions for at least six weeks (Brown et al., 2018).Transmission is by oro-fecal route.
GfCoV like TCoV is shed in faeces and the development of clinical signs in infected guinea-fowl flocks suggest that this virus also spread easily. To date no experimental data on transmission rates and routes are available.
-
Pathogenic life cycle stages
Not applicable.
-
Signs/Morbidity
IBV: In young birds (e.g. broilers) clinical signs most commonly seen include respiratory problems (conjunctivitis, rales), depression, drop in feed consumption and growth. Morbidity is usually 100%. Depending on the circumstances, secondary bacterial infections will manifest and contribute to the increased mortality and condemnation rates. Some isolates of IBV have a particular tropism for the kidney and are often associated with higher rates of mortality due to nephritis and wet litter (drop in feed consumption increase of water consumption).In layers and breeders, a drop in feed consumption, egg production and egg quality (including hatchability for breeders) can occur and this is usually more notable in older birds. Clinical signs suggestive of respiratory or renal disease are usually rare.
TCoV: Acute, highly contagious enteric disease characterized by depression, anorexia, diarrhea and decreased weight gain. Morbidity is 100% and turkey breeders show a drop in egg production.
GfCoV: Fulminating enteritis, severe depression, anorexia diarrhea and decreased weight gain, often combined with high mortality. Morbidity is 100%.
-
Incubation period
IBV: Respiratory signs can appear within 1-2 days post infection
TCoV: 2-3 days
GfCoV: No data available.
-
Mortality
IBV: Mortality due to IBV infections that have not been complicated due to secondary infections is usually low except when nephritis occurs in very young birds without sufficient protection.
TCoV: Uncomplicated infections usually are not associated with mortality. Under field conditions, mortality can be high depending on the age of the birds, co-infections and differences in management practices.
GfCoV: Unknown, no controlled experimental infections performed to date. Mortality due to the fulminating enteritis and complicating factors can reach 20% per day (Liais et al., 2014b).
GAP :
Controlled studies are required to gain knowledge on the pathogenicity of strains of GfCoV in absence of complicating factors.
-
Shedding kinetic patterns
IBV: Shedding of IBV occurs via aerosol and faeces. Depending on the strain involved and the level of protection of the infected chicken, shedding by the respiratory tract varies from 1 to a few weeks. The length of faecal shedding depends on the strain and level of protection; it varies from 1-2 weeks to a few months.
TCoV: Shedding in faeces can occur for a number of weeks up to 2 months.
GfCoV: Unknown.
GAP :
Shedding kinetics of IBV can vary significantly between different strains and serotypes. There is less information about the shedding kinetics of different TCoV strains and hardly any data on shedding of GfCoV.
-
Mechanism of pathogenicity
N.A.
-
Zoonotic potential
-
Reported incidence in humans
None.
-
Risk of occurence in humans, populations at risk, specific risk factors
Not applicable.
-
Symptoms described in humans
None.
-
Estimated level of under-reporting in humans
None.
-
Likelihood of spread in humans
Not applicable.
-
Impact on animal welfare and biodiversity
-
Both disease and prevention/control measures related
IBV: In most cases animals recover from infection after 1-2 weeks but this can vary with strains and type of bird (broiler, layer, breeder, etc), involvement of co-infections and housing conditions. Thus, some IBV infections will have more impact on welfare than others. Prevention and control measures through non-invasive vaccination, reduced numbers of birds, and strict hygiene commonly only improve animal welfare.
TCoV and GFCoV: The multifactorial enteric diseases involving these viruses can result in high mortality. However, these cases are uncommon.In line with IBV, prevention and control measures through reduced numbers of birds and strict hygiene only improve animal welfare.
-
Endangered wild species affected or not (estimation for Europe / worldwide)
No reports of AvCoVs having an impact on endangered wild species.
-
Slaughter necessity according to EU rules or other regions
Slaughter is not necessary according to EU rules.
-
Geographical distribution and spread
-
Current occurence/distribution
IBV is widespread worldwide. In many areas, a number of genotypes are present. Some genotypes are present in a number of continents (like Mass, QX, 793B), others are locally restricted.
-
Epizootic/endemic- if epidemic frequency of outbreaks
IBV: This virus is endemic, commercial poultry is vaccinated against IBV globally. In case of an introduction of a antigenetically new strain for which the used vaccination program does not raise sufficient protection, such a strain can spread rapidly through the region.
TCoV: This virus is found in most areas where turkeys are farmed.
GfCoV: The frequency of outbreaks is unknown as there are only few studies. The virus has only been described in 2010. Associated fulminating disease in Guinea fowl is uncommon.
-
Seasonality
IBV: Infections occur the entire year but clinical problems in the form of respiratory disease are seen more in seasons where climate control is more difficult (restricted ventilation of the house in case of cold, seasons with high variation in day-and-night temperature.
TCoV: Infections occur during the whole year.
GfCoV: As yet unknown, very few studies.
-
Speed of spatial spread during an outbreak
IBV: IBV is very infectious enveloped virus. It can spread by air but not very far due to the sensitivity to inactivation. Spreading by transport of infected birds or feces can cause spread over bigger distances. The extent of spatial spread is also depending on the success of vaccinations and vaccination programs on the farms around the outbreak.
TCoV and GfCoV: No vaccines are available for TCoV and GfCoV, the viruses can spread in an area depending on the level of biosecurity.
-
Transboundary potential of the disease
Occurrence of IBV, TCoV and GfCoV strains is often transboundary.
-
Route of Transmission
-
Usual mode of transmission (introduction, means of spread)
IBV: Principally, direct contact of infected birds with susceptible birds. Aerosol and or oro-faecal transmission.
TCoV: Principally, direct contact of infected birds with susceptible birds. oro-faecal transmission.
GfCoV: Most likely direct contact of infected birds with susceptible birds and oro-faecal transmission due to the enteric nature of the virus and its isolation from intestinal tissues and content. However, as of yet there is no experimental data.
-
Occasional mode of transmission
Vertical transmission of IBV seems to be very uncommon but not impossible (Cook & Garside, 1967). Also see Section “Reservoir (animal, environmental)“.
-
Conditions that favour spread
Poor hygiene, high density flocks, poor vaccination (in the case of IBV) may complicate pathogen spread.
-
Detection and Immune response to infection
-
Mechanism of host response
For young birds, the local immunity is the major means of protection. Neutralizing antibodies can be detected in the blood from 2-3 weeks after infection (De Wit, 2000). In young birds, the correlation between humeral antibodies and protection is not strong, seronegative birds can be well protected post vaccination. For layers, there is a much higher correlation between detection of hemagglutinating or neutralizing antibodies against the challenge strain and level of protection against a drop in egg production post challenge (Box et al., 1988).
-
Immunological basis of diagnosis
For IBV, many antibody tests are available. No commercial tests are available for detection of antibodies to TCoV and GfCoV. TCoV ELISA’s have been described. Some use IBV as antigen, others recombinant TCoV nucleoprotein or spike protein (Guy, 2020).
-
Main means of prevention, detection and control
-
Sanitary measures
CoV’s are enveloped viruses and therefore sensitive to treatment with soaps and disinfectants.
-
Mechanical and biological control
Cleaning, disinfection and biosecurity.
-
Diagnostic tools
Many diagnostic tests are available for IBV. For TCoV, PCR tests are available, the availability of antibody test is limited due to the lack of commercially available ELISAs.For GfCoV, little diagnostics are available due to the small market.
-
Vaccines
For IBV, live attenuated and inactivated vaccines are widely used (see section 2). For TCoV and GfCoV, no vaccines are available.
-
Therapeutics
There are no treatments available.
-
Biosecurity measures effective as a preventive measure
IBV, TCoV and GfCoV can be spread by infected manure and fomites. Short distance airborne infections are possible for IBV.
-
Border/trade/movement control sufficient for control
IBV is endemic worldwide.
-
Prevention tools
Vaccination is needed to control IBV.
-
Past experiences on success (and failures) of prevention, control, eradication in regions outside Europe
Eradication of IBV and TCoV has never been successful, prevalence is also far too high.
-
Costs of above measures
Eradication would be extremely costly due to the high prevalence.
-
Disease information from the WOAH
-
Disease notifiable to the WOAH
No.
-
WOAH disease card available
No.
-
WOAH Terrestrial Animal Health Code
-
WOAH Terrestrial Manual
-
Socio-economic impact
-
Zoonosis: impact on affected individuals and/or aggregated DALY figures
IBV, TCoV and GfCoV are not zoonotic.
-
Zoonosis: cost of treatment and control of the disease in humans
IBV, TCoV and GfCoV are not zoonotic.
-
Direct impact (a) on production
In general, both IBV, TCoV and GfCoV are capable of having a major impact on production of chicken, turkey and guinea fowl, respectively.
According to the World Bank, IBV is the second most damaging virus for the global poultry production after Avian Influenza (WorldBank & TAFS-Forum, 2011).
-
Direct impact (b) cost of private and public control measures
No direct impact of public control measures.Biosecurity measures need to be in place for a number of diseases and not just CoVs including all-in, all-out principles, change of clothes and boots, strict disinfection regimens, limit to visitors, disinfection of incoming vehicles.Costs of vaccination for IBV. A very high percentage of poultry flocks worldwide are vaccinated for IBV.
-
Indirect impact
Indirect impact mainly by disruption of production.
-
Trade implications
-
Impact on international trade/exports from the EU
Very limited.
-
Impact on EU intra-community trade
Very limited.
-
Impact on national trade
Very limited.
-
Main perceived obstacles for effective prevention and control
- High strain variation, presence of multiple variants in the same area at the same time, development of new variants.
- Demand for more knowledge required for the development of a new generation of vaccines that induce broad protection.
- No vaccines available for TCoV and GfCoV.
- Differences in the sensitivity of diagnostic assays, new strains might be missed.
- Global travel and trade enhances unintended introduction in areas were certain AvCoVs are not present.
- Movement of infected birds may enhance risk of introduction on a farm or in an area.
-
Main perceived facilitators for effective prevention and control
- IBV, TCoV and GfCov are important pathogens that can cause a lot of damage when uncontrolled and vaccination costs may be high (for IBV).
- Animal welfare considerations.
- Food security considerations.
- Production facilities.
- Early and proper diagnosis (routine surveillance) can result in better vaccination programs and lower loss of animals.
-
Links to climate
Seasonal cycle linked to climate
AvCoV Infections can occur during the whole year but clinical problems in the form of respiratory disease are seen more in seasons in when climate control is more difficult (restricted ventilation of the house in case of cold, seasons with high variation in day-and-night temperature.
-
Distribution of disease or vector linked to climate
IBV, TCoV and GfCoV are seen in all climates.
-
Outbreaks linked to extreme weather
Not relevant.
-
Sensitivity of disease or vectors to the effects of global climate change (climate/environment/land use)
Not relevant for the viruses themselves. However, the severity of infection also depends on the occurrence of co-infections. Several co-infections such as Coryza are sensitive to climate change.
Risk
-
- Live attenuated vaccines have shown to be far more effective than inactivated vaccines in young birds. In layers, inactivated vaccines are beneficial but only after a proper live priming. Live vaccines can spread and make the birds more susceptible for secondary bacterial infections.
Main critical gaps
-
- Inactivated vaccines for IBV that are capable of inducing protective immune responses equal to that of current live attenuated IBV vaccines.
- What is the basis of cross-protection for IBV.
- Rationally designed live attenuated marker vaccines for IBV that induce broad range protection.
- Cost effective, reliable molecular tests for full S1 genotyping of IBV.
- IBV strain specific ELISAs.
- Knowledge on the host range of the different AvCoVs.
- TCoV GfCoV specific ELISA tests are required to perform prevalence studies on these viruses.
- Koch’s postulates need addressing for TCoV and GfCoV.
- Relevance of IBV-like viruses in wild birds for poultry.
Conclusion
-
While it is likely that improvements in CoV vaccines and diagnostic tools could be achieved, new approaches should be focussed on delivering broad protection and identifying the optimal diagnostic window.
AvCoVs, continue to cause severe losses to the poultry industry despite (in the case of IBV) the use of different vaccines and vaccine programmes over the last 70 years. AvCoVs seem to have a strong capacity for rapid evolution and perhaps this is why they continue to cause problems. However, fundamental studies (epidemiological and experimental) unravelling how and why AvCoVs, especially IBV, can create such diversity are lacking. Thus more AvCoV studies are required that focus on genomic evolution, its dynamics and the driving factors involved (environmental, physical etc) so that better control measures can be conceived.
To help answer these questions the developments in molecular and serological laboratory tests suggested above would be very helpful.
Sources of information
-
Expert group composition
Paul Brown, French Agency for Food, Environmental and Occupational Heath Safety (ANSES), France – [Leader]
Sjaak De Wit, Royal GD and Veterinary Faculty of Utrecht University, The Netherlands
Tanja Opriessnig, The Roslin Institute, University of Edinburgh, UK and Iowa State University, USA
-
Date of submission by expert group
09-November-2020
-
References
Box, P. G., Holmes, H. C., Finney, P. M., & Froymann, R. (1988). Infectious bronchitis in laying hens: the relationship between haemagglutination inhibition antibody levels and resistance to experimental challenge. Avian Pathology, 17, 349-361.
Britton, P., Armesto, M., Cavanagh, D., & Keep, S. (2012). Modification of the avian coronavirus infectious bronchitis virus for vaccine development. Bioeng Bugs, 3, 114-119.
Brown, P. A., Touzain, F., Briand, F. X., Gouilh, A. M., Courtillon, C., Allee, C., Lemaitre, E., De Boisseson, C., Blanchard, Y., & Eterradossi, N. (2016). First complete genome sequence of European turkey coronavirus suggests complex recombination history related with US turkey and guinea fowl coronaviruses. Journal of General Virology, 97, 110-120.
Cao, H. P., Wang, H. N., Yang, X., Zhang, A. Y., Li, X., Ding, M. D., Liu, S. T., Zhang, Z. K., & Yang, F. (2013). Lactococcus lactis anchoring avian infectious bronchitis virus multi-epitope peptide EpiC induced specific immune responses in chickens. Biosci Biotechnol Biochem, 77, 1499-1504.
Cao, H. P., Wang, H. N., Zhang, A. Y., Ding, M. D., Liu, S. T., Cheng, H., Zhou, Y. S., & Li, X. (2012). Expression of avian infectious bronchitis virus multi-epitope based peptide EpiC in Lactococcus lactis for oral immunization of chickens. Biosci Biotechnol Biochem, 76, 1871-1876.
Cavanagh, D. (2005). Coronaviruses in poultry and other birds. Avian Pathology, 34, 439-448.
Cavanagh, D., Casais, R., Armesto, M., Hodgson, T., Izadkhasti, S., Davies, M., Lin, F., Tarpey, I., & Britton, P. (2007). Manipulation of the infectious bronchitis coronavirus genome for vaccine development and analysis of the accessory proteins. Vaccine, 25, 5558-5562.
Chen, H. W., Huang, C. Y., Lin, S. Y., Fang, Z. S., Hsu, C. H., Lin, J. C., Chen, Y. I., Yao, B. Y., & Hu, C. M. (2016). Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials, 106, 111-118.
Cook, J. K. A., & Garside, J. S. (1967). A study of the infectious bronchitis status of a group of chicks hatched from infectious bronchitis infected hens. Research in Veterinary Science, 8, 74-82.
De Wit, J. J. (2000). Detection of infectious bronchitis virus. Avian Pathology, 29, 71-93.
Falchieri, M., Lupini, C., Cecchinato, M., Catelli, E., Kontolaimou, M., & Naylor, C. J. (2013). Avian metapneumoviruses expressing Infectious Bronchitis virus genes are stable and induce protection. Vaccine, 31, 2565-2571.
Gomes, D. E., Hirata, K. Y., Saheki, K., Rosa, A. C., Luvizotto, M. C., & Cardoso, T. C. (2010). Pathology and tissue distribution of turkey coronavirus in experimentally infected chicks and turkey poults. Journal of Comparative Pathology, 143, 8-13.
Guionie, O., Courtillon, C., Allee, C., Maurel, S., Queguiner, M., & Eterradossi, N. (2013). An experimental study of the survival of turkey coronavirus at room temperature and +4 degrees C. Avian Pathology, 42, 248-252.
Guo, Z., Wang, H., Yang, T., Wang, X., Lu, D., Li, Y., & Zhang, Y. (2010). Priming with a DNA vaccine and boosting with an inactivated vaccine enhance the immune response against infectious bronchitis virus. J Virol Methods, 167, 84-89.
Guy, J. S. (2020). Turkey Coronavirus Enteritis. In D. E. Swayne, Boulianne, M., McDougald, L.R., Nair, V., Suarez, D.L. (Ed.), Diseases of Poultry (14th ed., Vol. I, pp. 402-408). Hoboken, NJ, USA: Wiley-Blackwell.
Jackwood, M., & de Wit, J. J. (2020). Infectious Bronchitis. In D. E. Swayne, Boulianne, M., McDougald, L.R., Nair, V., Suarez, D.L. (Ed.), Diseases of Poultry (14th ed., Vol. I, pp. 167-188). Hoboken, NJ, USA: Wiley-Blackwell.
Johnson, M. A., Pooley, C., Ignjatovic, J., & Tyack, S. G. (2003). A recombinant fowl adenovirus expressing the S1 gene of infectious bronchitis virus protects against challenge with infectious bronchitis virus. Vaccine, 21, 2730-2736.
Kampf, G., Todt, D., Pfaender, S., & Steinmann, E. (2020a). Corrigendum to "Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents" [J Hosp Infect 104 (2020) 246-251]. J Hosp Infect.
Kampf, G., Todt, D., Pfaender, S., & Steinmann, E. (2020b). Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect, 104, 246-251.
Kapczynski, D. R., Hilt, D. A., Shapiro, D., Sellers, H. S., & Jackwood, M. W. (2003). Protection of chickens from infectious bronchitis by in ovo and intramuscular vaccination with a DNA vaccine expressing the S1 glycoprotein. Avian Dis, 47, 272-285.
Liais, E., Croville, G., Mariette, J., Delverdier, M., Lucas, M. N., Klopp, C., Lluch, J., Donnadieu, C., Guy, J. S., Corrand, L., Ducatez, M. F., & Guerin, J. L. (2014a). Novel avian coronavirus and fulminating disease in guinea fowl, France. Emerg Infect Dis, 20, 105-108.
Liais, E., Croville, G., Mariette, J., Delverdier, M., Lucas, M. N., Klopp, C., Lluch, J., Donnadieu, C., Guy, J. S., Corrand, L., Ducatez, M. F., & Guerin, J. L. (2014b). Novel avian coronavirus and fulminating disease in guinea fowl, France. Emerging infectious diseases, 20, 105-108.
Panigrahy, B., Naqi, S. A., & Hall, C. F. (1973). Isolation and characterization of viruses associated with transmissible enteritis (bluecomb) of turkeys. Avian Dis, 17, 430-438.
Ritchie, A. E., Deshmukh, D. R., Larsen, C. T., & Pomeroy, B. S. (1973). Electron microscopy of coronavirus-like particles characteristic of turkey bluecomb disease. Avian Dis, 17, 546-558.
Shalk, A. F., & Hawn, M. C. (1931). an apparently new respiratory disease of baby chicks. J.Am.Vet.Med.Assoc 78, 413-422.
Sjaak de Wit, J. J., Cook, J. K., & van der Heijden, H. M. (2011). Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol, 40, 223-235.
Tan, B., Wang, H., Shang, L., & Yang, T. (2009). Coadministration of chicken GM-CSF with a DNA vaccine expressing infectious bronchitis virus (IBV) S1 glycoprotein enhances the specific immune response and protects against IBV infection. Arch Virol, 154, 1117-1124.
Tan, L., Wen, G., Qiu, X., Yuan, Y., Meng, C., Sun, Y., Liao, Y., Song, C., Liu, W., Shi, Y., Shao, H., & Ding, C. (2019). A Recombinant La Sota Vaccine Strain Expressing Multiple Epitopes of Infectious Bronchitis Virus (IBV) Protects Specific Pathogen-Free (SPF) Chickens against IBV and NDV Challenges. Vaccines (Basel), 7.
Tang, M. J., Wang, H. N., Zhou, S., Huang, Y., & Liu, P. (2007). [Potent immune responses elicited by a bicistronic IBV DNA vaccine expressing S1 and IL-2 gene]. Wei Sheng Wu Xue Bao, 47, 1055-1059.
Tian, L., Wang, H. N., Lu, D., Zhang, Y. F., Wang, T., & Kang, R. M. (2008). The immunoreactivity of a chimeric multi-epitope DNA vaccine against IBV in chickens. Biochem Biophys Res Commun, 377, 221-225.
Valastro, V., Holmes, E. C., Britton, P., Fusaro, A., Jackwood, M. W., Cattoli, G., & Monne, I. (2016a). S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect Genet Evol, 39, 349-364.
Valastro, V., Holmes, E. C., Britton, P., Fusaroa, A., Jackwood, M. W., Cattoli, G., & Monne, I. (2016b). S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification Infection, Genetics and Evolution, 39 349-364.
WorldBank, & TAFS-Forum. (2011). World Livestock Disease Atlas, A Quantitative Analysis of Global Animal Health Data (H. S. N. The World Bank, Washington, DC 20433, USA Ed.).
Yan, F., Zhao, Y., Hu, Y., Qiu, J., Lei, W., Ji, W., Li, X., Wu, Q., Shi, X., & Li, Z. (2013). Protection of chickens against infectious bronchitis virus with a multivalent DNA vaccine and boosting with an inactivated vaccine. J Vet Sci, 14, 53-60.
Yang, T., Wang, H. N., Wang, X., Tang, J. N., Lu, D., Zhang, Y. F., Guo, Z. C., Li, Y. L., Gao, R., & Kang, R. M. (2009). The protective immune response against infectious bronchitis virus induced by multi-epitope based peptide vaccines. Biosci Biotechnol Biochem, 73, 1500-1504.
