Diseases
African Horse Sickness
Chapter select
Control Tools
-
Diagnostics availability
-
Commercial diagnostic kits available worldwide
Commercial kits for the diagnosis of AHS are available:
- RT-PCR serogroup specific
- double antibody sandwich Enzyme Immuno-sorbent assay (ELISA) capable to detect AHS antigen
- a blocking-ELISA and a flow lateral assay to detect AHSV antibodies in serum samples. Both kits are able to detect the antibodies against the antigenically conserved viral protein 7 (VP7) of the AHSV. The protein is in the outer core of the viral capsid and is the group specific antigen.List of animal health diagnostics (Diagnostics for animals) https://diagnosticsforanimals.com/list-of-animal-health-diagnostics/
GAP:
The blocking ELISA has been recently validated according to the OIE validation pathway up to stage 3 (determination of the reproducibility) through an international collaborative trial among reference and specialist AHS laboratories worldwide. Such validation data are not available for the other two serological assays.
Need for validated and harmonised serological and virological assays.
-
Commercial diagnostic kits available in Europe
A sandwich enzymatic immunoassay capable to detect AHS antigen is commercially available.Different kits for serological diagnosis are commercially available: c-ELISA and a flow lateral assay to detect AHSV antibodies. Both products use the VP7 recombinant protein, one of the mayor viral proteins as well as the most antigenic and conserved within the 9 different serotypes. Regardless the serotype involved, it has not been found any infected or vaccinated animal without antibodies to this protein.
GAPS:
Only one manufacturer for the serological kits; additional commercial ELISA kits from different companies would be advantageous to improve the serological diagnostic capacities, the robustness of the results and the availability of kits if any outbreak occurs.
No commercial kits are available for the molecular diagnosis of AHSV.Need for validated and harmonised serological and virological assays.
-
Diagnostic kits validated by International, European or National Standards
The OIE validation pathway identifies four stages: Stage 1, determination of analytical characteristics (analytical sensitivity, specificity and repeatability estimates); Stage 2, determination of diagnostic characteristics (diagnostic sensitivity and specificity estimates); Stage 3, determination of reproducibility estimates; and Stage 4, declaration of fitness for purpose and international recognition (OIE, 2013. Manual of Diagnostics and Vaccines for Terrestrial Animals, Chapter 1.1.6).The blocking ELISA based on the AHSV VP7 antigen (VP7 ELISA) is one of the OIE prescribed test for International trade and is widely used amongst OIE reference centres and veterinary diagnostic laboratories. It has been extensively used in the past years in proficiency tests within European Union laboratories and identified as suitable for AHS surveillance. This assay commercially available, has been recently validated up to stage 3 of the OIE pathway. To this aim a study was funded by the OIE (AD/SR/2015/1885) and a ring trial, which included the participation of OIE Reference Laboratories and other specialist diagnostic laboratories, was set up to test a panel of field sera of different epidemiological origin and experimental sera from horses infected with AHSV and / or vaccinated with live attenuated, inactivated or recombinant AHSV vaccines.
The indirect ELISA, the competitive ELISA and the VP7-Blocking ELISA are the serological tests prescribed for the control of movement and importations to the European Union, according to requirements of Directive 2009/156/EC, annex IV (European Union, 2009).
In 2015 the OIE Reference Laboratories for AHS carried out an international ring trial to gather information on the performance of the different methods used in the main AHSV diagnostic laboratories. Ten different RT-PCR protocols were evaluated. Two real-time RT-PCR methods, published by Agüero et al (2008) and Guthrie et al. (2013) respectively were able to correctly detect all the representative strains included in the international trial with a high sensitivity. These methods are recommended for certification of individual animals prior to movements.
-
Diagnostic method(s) described by International, European or National standards
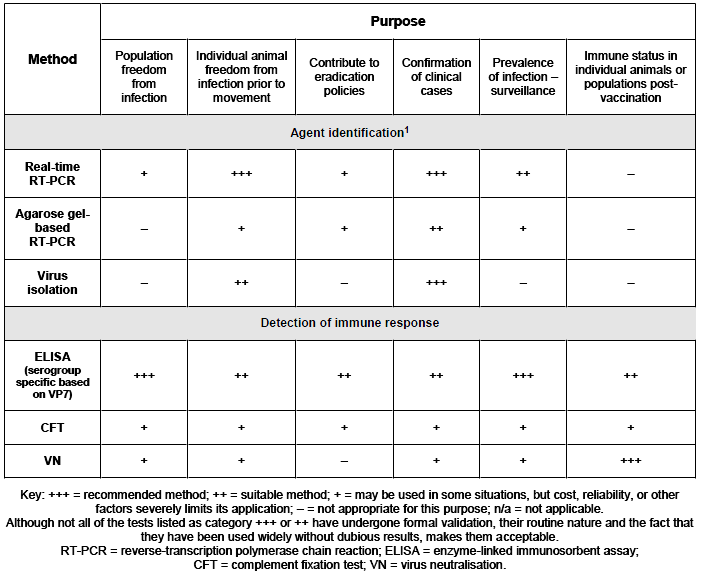
The table summarized the diagnostic test available for the diagnosis of AHS and their purpose (OIE, Terrestrial Manual, 2019. Chapter 3.5.1 African horse sickness. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.05.01_AHS.pdf).
However some clarifications are provided below:
- currently, RT-PCR methods (gel based or Real time tests) are the first choice for AHSV detection. Once the disease has been confirmed, the virus needs further characterization, primarily the serotype identification. To this aim several molecular tests have been published providing a rapid typing method for AHSV in biological samples. However the virus neutralization test (VN) should be considered as the gold standard for serotyping of AHSV isolates due to the genetic variability of the genome segment coding for the VP2, the segment targeted by the molecular typing assays;
- the complement fixation test (CFT) has been progressively replaced by the ELISA tests which are more sensitive and easy to standardize and not affected by the anti-complementary activity shown by a certain amount of tested sera;
- the gold standard for the identification of AHS serotypes in suspicious sera is VN. With this technique, serotype-specific antibodies are detected. The VN test may have additional value in epidemiological surveillance and transmission studies, mainly in endemic areas where multiple serotypes are likely to be present.
The indirect ELISA, the competitive ELISA and the VP7-Blocking ELISA are the serological tests prescribed for the control of movement and importations to the European Union, according to requirements of Directive 2009/156/EC, annex IV (European Union, 2009).
-
Commercial potential for diagnostic kits worldwide
Low
-
DIVA tests required and/or available
No serological/molecular DIVA tests are currently available but they are needed. An ELISA test capable to detect the antibodies specific for the Non-Structural protein 3 (NS3) of the AHSV4 has been developed and used to distinguish infected horses from those immunized with an inactivated purified vaccine.
GAP:
The capability to Differentiating Infected from Vaccinated Animals (DIVA) requests the use of both marker vaccines and diagnostic test specifically designed to target the antibody response evoked by the vaccine. Once available, the diagnostic test needs to be validated and harmonized among laboratories.
-
Opportunities for new developments
Molecular detection of AHSV is the best alternative for rapid diagnosis of AHSV. RT-PCR is a sensitive and rapid method for detecting AHSV nucleic acids during either the incubation period at the start of an African horse sickness (AHS) epizootic, or for epidemiological investigations in species where clinical signs may not be apparent.
It is also known that subclinical infections are relatively common amongst vaccinated horses in endemic Countries emphasizing the importance of using the real time PCR assay to screen the horse population. The occurrence of AHSV subclinical infections among immunised horses raises some concerns whether clinical surveillance would always be sufficient in high risk areas, especially if live-attenuated vaccine is used close by.
GAP:
A of Pen-side diagnostic assay is commercially available but its performances need to be fully evaluated.
-
Vaccines availability
-
Commercial vaccines availability (globally)
There are a number of live attenuated vaccines against AHS manufactured in the world. Most of them commercialise in mono or polyvalent vaccines to be used in endemic situations. Currently, there are no commercially available inactivated or recombinant vaccines but there are some locally killed vaccines available in some countries
GAP:
Establishment of well characterized safe and effective AHS vaccine banks with long shelf-live for international use
-
Commercial vaccines authorised in Europe
No vaccine for AHS is currently licensed in the EU.
An inactivated vaccine was developed and its efficacy assessed under laboratory and field conditions. Despite the good results obtained with this vaccine, it is no longer available. The main advantage of this vaccine was that it allows to differentiate between infected and vaccinated animals. This was based in the lack of NS3 protein in the vaccine strain.
GAP :
Registration process long, heavy and complex.
-
Marker vaccines available worldwide
None.There are different platform technologies suitable to develop AHS DIVA vaccines: sub-unit adjuvanted vaccines, modified Vaccinia Ankara (MVA), Canarypox virus, live attenuated vaccines and killed vaccines developed by reverse genetics.
GAP :
Process to authorise/commercialise the experimental vaccine candidates is long and complex.
-
Marker vaccines authorised in Europe
None
-
Effectiveness of vaccines / Main shortcomings of current vaccines
There is some concern about the safety and efficacy of the live vaccines that are produced in various countries across Africa.
A) Do not protect against all variants of the virus.
B) The use of a modified live vaccine for AHS (such as the one being produced by OBP) carries a risk of vaccine virus reversion to wild type and reassortment with vaccine and field strains producing a viral progeny with increased patogenicity.
C) Other problems include:- Occurrence of vaccine reactions, including death, variable response to vaccination, and the difficulty immunizing young horses due to interference from maternal immunity.
D) Cannot differentiate vaccinated from infected equidae.
E) Requires 2 or 3 doses but does give lifelong immunity.
F) Subclinical infection in immunized horses.
GAP:
Evaluate the risk of transfer of the modified-live vaccine strain into the eco-system.
Cross-serotype protection would be welcomed in areas where multiple serotypes are circulating.
-
Commercial potential for vaccines
Low purely required as a precautionary measure in the event of an outbreak occurring in Europe
GAP:
Lack of an economic model to justify the development of AHS vaccines
-
Regulatory and/or policy challenges to approval
Timescale
GAP:
Applying normal rules for licensing will not allow the development of cost-effective emergency veterinary vaccines in time. In order to reduce time-to-market, specific exemptions for well established non-replicative vaccine platforms are reasonable options to be considered
Possibility for Multi-strain license
Impact of vaccination on international trade
Risk management of AHS is a shared responsibility of the pharmaceutical industry and all stakeholders
-
Commercial feasibility (e.g manufacturing)
Feasible
GAP :
Commercial feasibility is in general high which does not necessarily mean that vaccine is available in a short time if there is no stock available.
-
Opportunity for barrier protection
Yes
-
Opportunity for new developments
To overcome the current problems with the live vaccines or preferably to develop new recombinant vaccines
-
Pharmaceutical availability
-
Current therapy (curative and preventive)
No efficient treatment
-
Future therapy
Non apparent at present apart form possible development of anti virals or immune stimulants.
-
Commercial potential for pharmaceuticals
Low
-
Regulatory and/or policy challenges to approval
Not applicable
-
Commercial feasibility (e.g manufacturing)
Low
-
Opportunities for new developments
None at present
-
New developments for diagnostic tests
-
Requirements for diagnostics development
New developments in molecular diagnostic methods to improve sensitivity of AHSV detection
GAP:
Need for validated and harmonized diagnostic assays.Need for molecular and serological test for AHSV typing as an alternative to the VN test.
-
Time to develop new or improved diagnostics
The time necessary to develop a novel assay varies according to the technique and the resources available. However, beside development, the process of full validation of the test is usually the longest step.
GAP :
Need to improve/promote cooperation between diagnostic/research labs and commercial companies in order to combine resources, share relevant expertise, reduce cost and speed the process.
-
Cost of developing new or improved diagnostics and their validation
Medium to high depending to the test, the state of art etc.
GAP :
Need to improve/promote cooperation between diagnostic/research labs and commercial companies in order to combine resources, share relevant expertise, reduce cost and speed the process.
-
Research requirements for new or improved diagnostics
Understanding of the host pathogen interactions with the development of improved pen side test facilities.
-
Technology to determine virus freedom in animals
- Serology and PCR-diagnostics. Serotype-specific ELISAs and serotype-specific PCRs for all serotypes are useful to determine freedom for a respective serotype.
- Marker vaccines which allow DIVA strategy.
-
New developments for vaccines
-
Requirements for vaccines development / main characteristics for improved vaccines
The potential use of modern recombinant vaccines should be considered because of their safety and their role in distinguishing vaccinated from infected animals. No need for BSL-3 production facilities.Safe, not expensive, lifelong and cross protection.
GAP:
Availability of cross-reactive vaccines offering protection against multiple serotypes.
-
Time to develop new or improved vaccines
5 years
-
Cost of developing new or improved vaccines and their validation
Unknown
-
Research requirements for new or improved vaccines
Research is needed to develop vaccines that are safe and able to provide lifelong protection against all serotypes.
GAP:
Better understanding of the molecular biology of the virus and market demand for this type of AHS vaccine.
-
New developments for pharmaceuticals
-
Requirements for pharmaceuticals development
Not applicable
-
Time to develop new or improved pharmaceuticals
Not applicable
-
Cost of developing new or improved pharmaceuticals and their validation
High
-
Research requirements for new or improved pharmaceuticals
None at present
Disease details
-
Description and characteristics
-
Pathogen
Viscerotropic virus, family Reoviridae, genus Orbivirus. The AHS virus genome is composed of ten double-stranded RNA segments numbered from 1 to10 in order of their migration in PAGE. These segments code for the seven structural proteins (VP1 to 7) and four non-structural proteins (NS1, NS2, NS3 and NS3A). Virions are non-enveloped particles of approximately 70 nm in diameter, structured as a two- layered icosahedral capsid composed of 32 capsomeres. The core of the virion is formed by two major proteins, VP3 and VP7, highly conserved among the nine AHSV serotypes and responsible for the group specific typing, and three minor proteins, VP1, VP4 and VP6. An outer capsid composed of VP2 and VP5 is mainly responsible for serotype antigenic characteristics; neutralizing epitopes are mainly located in VP2 .
GAP:
Potential for the identification of novel serotypes using the new generation diagnostic assays and sequencing technologies.
-
Variability of the disease
There are nine different types of AHS virus (type 1-9) with marked genetic heterogeneity being recognised within each type. No new serotypes have been detected in recent years. There is partial antigenic relationship between these serotypes, with cross-reactivity of homologous antiserum to other subtypes in VN assay (cross-neutralization between types 1 and 2, 3 and 7, 5 and 8, and 6 and 9). This cross- protection has been also observed in the field, and is a common practice in South Africa that polyvalent vaccines do omit some serotypes taking into account the cross- protection offered by other types. The severity of clinical disease varies between each strain but it is likely that an outbreak of AHS involving any serotype could have a major impact on the equine population in a country which has not experienced the disease.
GAP:
Need for sequencing of circulating strain to investigate the molecular epidemiology of the disease, to assess the diagnostic capabilities of the molecular test in use and to investigate the potential for vaccine strains circulation, reversion to virulence and/or reassortment.
-
Stability of the agent/pathogen in the environment
Temperature: Inactivated by 50°C/3 hours; 60°C/15 min pH: Survives between pH 6.0 and 12.0 Chemicals: Inactivated by ether and ß-propiolactone 0.4% Disinfectants: Inactivated by formalin 0.1%/48 hours. Also phenol and iodophores Survival:Survives at 37°C/37 days
Freezing at temperatures between –20 °C and –30 °C causes significant loss of titre
GAP:
Understanding the over wintering mechanism(s) in the host, vector and/or environment including potential differences between species and distinguished ecological zones.
-
Species involved
-
Animal infected/carrier/disease
Usual hosts: horses, mules, donkeys, zebra. Donkeys and zebras are relatively resistant to AHS and will generally show less severe clinical signs than horses and ponies.
Occasional hosts: elephants, camels (Hyalomma dromedarii) and wild carnivores from ingestion of meat and organs from AHS-infected prey species. Zebras and elephants may be infected without showing signs of disease. Dogs have been known to be infected by eating infected horsemeat.
GAP:
Duration of viraemia in wild susceptible species and their infectivity for vector.Potential role of non-equine species on the epidemiology of AHS.
-
Human infected/disease
AHS does not affect humans, so there are no human or public health implications.
-
Vector cyclical/non-cyclical
At least two field vectors are involved in the transmission of the virus: Culicoides imicola and C. bolitinos. However, during the 1987–1990 outbreaks in Spain and Portugal, AHSV isolations were made from mixed pools of Culicoides consisting almost entirely of C. obsoletus and C. pulicaris without C. imicola. It is feasible that AHS virus could be transmitted by vectors other than C. imicola especially as is considered that the Culicoides species which are commonly found in northern Europe might be able to transmit AHS virus based on evidence from the transmission of the closely related Bluetongue virus.
GAP:
Need to understand the potential competence of different Culicoides species for AHSV in currently non-affected areas.
-
Reservoir (animal, environment)
It is likely that the virus persists (overwinters) in other, unknown species in Africa when the insect is not active. This explains why the disease does not persist in other countries following an outbreak.
GAP:
The potential mechanisms and likelihood of overwintering of AHSV in currently non-affected areas needs to be investigated.
-
Description of infection & disease in natural hosts
-
Transmissibility
The disease is not directly contagious between horses. AHS virus is transmitted when an infected competent vector (Culicoides spp.) feeds on a susceptible host.
GAP:
Vector competence of the European species of Culicoides.Investigate the potential for transplacental transmission.
-
Pathogenic life cycle stages
During its life cycle AHSV infects arthropod and vertebrate hosts. The virus has an enzootic cycle and is transmitted from arthropod vector to competent reservoir host.After bite from an infected Culicoides, the virus multiplies in endothelium of lymph capillary vessels and regional lymph nodes eliciting a primary viraemia. Dissemination to capillary vessels of many organs then occurs, mainly to the lungs, large intestine, and lymphoid organs, causing a secondary viraemia.
GAP:
Vector competence of the European species of Culicoides.
-
Signs/Morbidity
The clinical signs seen are different depending on what form of the disease is present.
- Subclinical form: fever (40-40.5°C) and general malaise for 1-2 days
- Subacute or cardiac form: fever (39-41°C), swelling of the supraorbital fossa, eyelids, facial tissues, neck, thorax, brisket and shoulders. Death usually within 1 week
- Acute respiratory form: fever (40-41°C), dyspnoea, spasmodic coughing, dilated nostrils with frothy fluid oozing out, redness of conjunctivae, death from anoxia within 1 week
- A mixed form (cardiac and pulmonary) occurs frequently: pulmonary signs of a mild nature that do not progress, oedematous swellings and effusions, death from cardiac failure, usually within 1 week
In the majority of cases, the subclinical cardiac form is suddenly followed by marked dyspnoea and other signs typical of the pulmonary form. A nervous form may occur, though it is rare. The severity of disease depends upon the virus strain and host species.
GAP:
Genetic determinants for pathogenicity.
-
Incubation period
Incubation period is usually 7-14 days, but may be as short as 2 days.
-
Mortality
Mortality due to AHS is related to the species of equidae affected and to the strain or serotype of the virus. In the most acute cases the mortality rate can be 95% with horses dying within a week. Mortality rate in horses is 70-95%, in mules it is around 50%, and in donkeys it is around 10%.
GAP:
Genetic determinants for pathogenicity.
-
Shedding kinetic patterns
According to OIE the infectious period is up to 40 days post infection although most animals are infectious to vectors for a shorter period: viraemia in horses may extend for as long as 18 days, but usually lasts for fewer days - about 4-8 days. In zebras and donkeys viraemia may last up to 28 days.
GAP:
Comparisons of virus isolation and molecular techniques for determination of the length of viraemia in horses are not available. As in the case of BTV in ruminants (EFSA, 2007), a much longer RT-PCR viraemia than virus isolation viraemia would be expected.
-
Mechanism of pathogenicity
Once in the host, AHS virus multiplies in the endothelium of lymph capillary vessels and regional lymph nodes eliciting a primary viraemia. Dissemination to capillary vessels of many organs then occurs, mainly to the lungs, large intestine, and lymphoid organs, causing a secondary viraemia.AHSV infects primarily endothelial cells and some cells of the monocyte-macrophage lineage and reticular cells in lymphoid organs. Replication in endothelial cells is followed by cellular damage with alteration of intercellular junctions, loss of endothelium, increased capullary permeability, and subendothelial deposition of cell debris and fibrin. Oedeman haemorrhages and microthrombi may be seen in many organs.AHSV grows in cells lining blood vessels of the lungs but damage to cells is not severe or extensive enough to cause the pathology observed!
GAP:
A better understanding of the pathogenesis is required.
-
Zoonotic potential
-
Reported incidence in humans
None.
-
Risk of occurence in humans, populations at risk, specific risk factors
None.
-
Symptoms described in humans
None.
-
Estimated level of under-reporting in humans
None.
-
Likelihood of spread in humans
None.
-
Impact on animal welfare and biodiversity
-
Both disease and prevention/control measures related
Clinical disease has a variable impact depending on species, immune status, the virulence, and genotype of the infecting virus strain.
-
Endangered wild species affected or not (estimation for Europe / worldwide)
Unlikely unless of the family Equidae.
-
Slaughter necessity according to EU rules or other regions
The Council Directive 92/35/EEC, of 29 April 1992, established the control measures to combat AHS once an outbreak of the disease is suspected. If one or more equidae (horses) on a holding are suspected of being infected with AHS, the official veterinarian must notify the competent authority and take a number of measures which may only be discontinued when the competent authority has confirmed that the presence of AHS is no longer suspected. Once the outbreak of AHS is confirmed, the veterinarian must have all infected equidae slaughtered and their carcases disposed of.
-
Geographical distribution and spread
Sub-Saharan Continental Africa, Near and Middle East, Spain, Portugal, Morocco
-
Current occurence/distribution
The disease is endemic in sub-Saharan Continental Africa from where it occasionally spreads to other suitable areas. A few outbreaks have occurred outside Africa, such as in the Near and Middle East (1959-63), in Spain (1966 serotype 9, 1987-90 serotype 4) and in Portugal (1989-serotype 4), and Morocco (1989 – 1991 serotype 4).
GAP:
Improvements to conduct and collation of surveillance in sub-Saharan and bordering region. Improved knowledge of the potential for indirect non-equine (trade) threats from affected areas.
-
Epizootic/endemic- if epidemic frequency of outbreaks
Variable.
-
Seasonality
The spread of disease is influenced by climatic conditions which favour the spread of carrier insects (vectors) including warm, moist weather and high rainfall, as well as spread by wind dispersal. In Europe cyclic outbreaks (usually during the late summer through late autumn) would coincide with the availability and abundance of the competent vector(s).
-
Speed of spatial spread during an outbreak
Variable
GAP:
Need for disease transmission modelling in currently non-affected areas.
-
Transboundary potential of the disease
The spread of disease is influenced by climatic conditions which favour the spread of carrier insects (vectors) including warm, moist weather and high rainfall, as well as spread by wind dispersal. In Europe cyclic outbreaks (usually during the late summer through late autumn) would coincide with the availability and abundance of the competent vector(s).
-
Route of Transmission
-
Usual mode of transmission (introduction, means of spread)
Usual mode of transmission: Culicoides spp., i.e. biological vector
-
Occasional mode of transmission
Occasional mode of transmission: mosquitoes - Culex, Anopheles and Aedes spp.; ticks - Hyalomma, RhipicephalusThe virus can also be mechanically transmitted through transfusion of infected blood products or through unhygienic practices (e.g. use of contaminated surgical equipment or hypodermic needles). It is unknown whether AHS can be transmitted by semen or ova from infected animals.
-
Conditions that favour spread
Any factor which favours the insect vector. Because of their small size, Culicoides generally do not fly in windy conditions, however they are easily spread over long distances by light winds. Consequently virus movement over long distances via windborne infected vectors has been suggested.
-
Detection and Immune response to infection
-
Mechanism of host response
Neutralisation.Immunity acquired after infection or induced by vaccination against one serotype is often ineffective or less effective in case of infection due to a different serotype.
GAP:
Increase understanding of the immune responses to AHSV to improve vaccination strategies: including identification of viral epitopes involved in type specific and cross-reactive immune reactions and protection.Characterize the role of cell-mediated immunity.
-
Immunological basis of diagnosis
Identification of neutralizing antibodies induced by VP2 and VP5 (serotype specific) or VP7 (serogroup specific).
GAP:
Development to serotype-specific ELISA assays.
-
Main means of prevention, detection and control
-
Sanitary measures
Effective control of AHS is based on four pillars:a) Availability of a safe efficacious vaccine; b) Control of movement of horses; c) Use of accurate and validated diagnostic methods; and d) Effective insect vector control procedures. Suspect cases should be placed under vector-protected conditions immediately to reduce transmission pending diagnosis. Control by slaughtering of affected horses and destruction of cadavers.Vector control (insecticides, repellents, screens)Identification of vaccinated horses.
-
Mechanical and biological control
The adoption of measures aimed at preventing horses being bitten by the infected biting midges, Culicoides represents a pillar of the disease control measuresEven if physical and chemical barriers as well as husbandry measures may help to prevent Culicoides attacks there is no mechanism of protection that will guarantee the horse will not be bitten. Housing horses in accommodation at times of peak midge activity will reduce the likelihood of midge attack but it is unlikely to fully protect against AHS. A combination of protection measures is recommended to be implemented in horse stables, at quarantine stations and in transport vehicles before and during transportation to reduce the impact of the disease during outbreaks.
GAP:
Development of more effective insecticides.
-
Diagnostic tools
Antibody Detection: ELISA, Virus neutralisation.
Antigen detection: Real time RT-PCR or conventional RT-PCR, type specific RT-PCR, virus isolation and direct ELISA.
Serogroup specific conventional and real-time RT-PCR assays are available.
GAP:
Need for harmonized and validated diagnostic serological and virological assays.Development of serotype specific ELISAs.Development of DIVA serology assays in combination with DIVA vaccine.
-
Vaccines
Three types of vaccine for AHS can be considered: live attenuated, inactivated and recombinant. For vaccination of non-infected horses, polyvalent vaccine or monovalent vaccine may be used. Monovalent is better when virus has been typed. In endemic areas vaccination of susceptible Equidae.
GAP:
Need for an effective and safe AHS vaccine that confers rapid and effective immunity, with the capacity to distinguish vaccinated animals by DIVA serological test.
-
Therapeutics
No successful treatment available for AHS.
GAP:
Efficient antivirals to limit the clinical signs.
-
Biosecurity measures effective as a preventive measure
Restrict movement of infected horses into new areas.No contact between vector and susceptible animals in infected areas.Quarantine in Culicoides proof stables.
GAP:
Effective insecticides.
-
Border/trade/movement control sufficient for control
Prevent the introduction of AHS into free areas. In Europe a protection zone of least 100 kilometers radius around and infected premises would be established. This, together with a surveillance zone of at least a further 50 kilometers, would have to remain in force for at least 12 months.
-
Prevention tools
No insecticide will guarantee protection against AHS and insecticide use against Culicoides may not provide a practical and cost-effective control measure in all cases. In some countries no insecticides are authorised for use specifically against Culciodes in any species.
-
Surveillance
Being an exotic disease, awareness and familiarity of owners, horse keepers and veterinarians with AHS clinical signs would facilitate early detection as a key limiting factor to potential dissemination of the disease.Knowledge of the presence and abundance of the Culicoides species.
GAP:
Need for DIVA assays.
-
Past experiences on success (and failures) of prevention, control, eradication in regions outside Europe
Early detection of index case, prompt notification, and action by the authorities, the proximity to/density of susceptible host population and the availability of competent vectors will have an impact on the incidence of the AHS.The most recent outbreak of AHS in Europe was reported in the Iberian Peninsula in 1990s. The AHS virus (type 4) was considered to be introduced to Spain by importation of zebras from Namibia in 1987. The disease was successfully contained and eradicated by a combination of disease control measures (i.e. destruction, movement control and vaccination).
GAP:
The time elapsing from incursion to vaccination can be significant in determining the impact of cuicoides-borne outbreaks.Modelling the use of vaccination as an effective control measure.
-
Costs of above measures
Costs of vaccine if applied. Cost of surveillance.
-
Disease information from the WOAH
-
Disease notifiable to the WOAH
Yes. AHS is the only equine disease for which the OIE provides recognition of disease freedom
-
WOAH disease card available
-
WOAH Terrestrial Animal Health Code
-
WOAH Terrestrial Manual
-
Socio-economic impact
-
Zoonosis: impact on affected individuals and/or aggregated DALY figures
None
-
Zoonosis: cost of treatment and control of the disease in humans
None
-
Direct impact (a) on production
High mortality and morbidity.
-
Direct impact (b) cost of private and public control measures
Cost of measures including vaccination and movment restrictions.
-
Indirect impact
Disrupt market for horse for sport and meat. Disruption to sports such as racing, trotting ,horse trials etc.
-
Trade implications
-
Impact on international trade/exports from the EU
AHS has a significant impact on international trade in equidae and their products; The severity of disease and the controls to monitor and restrict movement of horses could significantly affect the Equine Industry worldwide especially in racing.
-
Impact on EU intra-community trade
The severity of disease and the controls to monitor and restrict movement of horses could significantly affect the Equine Industry in Europe both for racing, companion animals and the trade in equine products.
-
Impact on national trade
The severity of disease and the controls to monitor and restrict movement of horses could significantly affect national movement of sport animals as well as food production for those countries which produce horsemeat.
GAP:
Need for sequencing of circulating strain to assess the diagnostic capabilities of the molecular test in use and to investigate the potential for vaccine strains circulation, reversion to virulence and/or reassortment.
-
Main perceived obstacles for effective prevention and control
Inadequate vaccine, differentiation of infected form vaccinated not possible. Attenuated vaccines may not be safe in AHS-free countries
-
Main perceived facilitators for effective prevention and control
Development of improved vaccines which are not a risk of reversion and give high levels of protection. Ease of application.
-
Links to climate
Seasonal cycle linked to climate
Epidemics of AHS tend to occur at cyclic intervals, and are often associated with drought followed by heavy rain presumably giving rise to large numbers of competent vectors.
-
Distribution of disease or vector linked to climate
Moist mild conditions and warm temperatures favour the presence of insect vectors.
-
Outbreaks linked to extreme weather
Not specifically.
-
Sensitivity of disease or vectors to the effects of global climate change (climate/environment/land use)
Predicted climate changes could create potential for northward distribution of the main known biological vector (i.e. Culicoides imicola) in Europe.There are also concerns that existing Culicoides spp that are present in Europe may favour the spread of the disease should it be introduced.
GAP:
Cullicoides species involved in AHSV transmission.Role of the climate change.
Risk
-
There is the potential for AHS to spread into Europe and new areas due to either the modification in the distribution of competent/potentially competent vectors or modification in vector competence due to climate changes.There are no AHS vaccines currently licensed in the EU. The vaccine commercially available, based on live attenuated strains, may not be safe in AHS-free countries due to the risk of transmission, reassortment and reversion to virulence. The time elapsing from incursion to vaccination can be significant in determining the impact of AHS outbreaks.
Main critical gaps
Conclusion
-
The genome sequencing of AHSV circulating strains should be encouraged in order to assess the diagnostic capabilities of the molecular test in use and to investigate the potential for vaccine strains circulation and/or reassortment.There are no authorised and safe vaccines along with tests to differentiate vaccinated form infected horses. The development of cross-protective AHSV vaccines with a long shelf life and that can provide rapid protection and be differentiated from natural infections during outbreaks is a major priority for research.There is the need to acquire information on the pathogenesis and ecology of the virus to model the possible pathways of introduction and dissemination of the AHS virus in naïve areas.
Sources of information
-
Expert group composition
F. Monaco, National Reference Laboratory for AHS, Istituto Zooprofilattico Sperimentale dell’Abruzzo e Molise “G. Caporale”, Italy – [Leader]
J.N. MacLachlan, School of Veterinary Medicine, University of California, USA.
P. Hudelet, Boehringer Ingelheim, France.
A. Lubisi, Onderstepoort Veterinary Institute, South Africa.
M. Aguero, EURL and OIE Reference Laboratory for AHS, Laboratorio Central de Sanidad Animal LCV- Spain.
A. Laddomada, Istituto Zooprofilattico Sperimentale della Sardegna “G. Pegreffi”, Italy.
P. Calistri, Istituto Zooprofilattico Sperimentale dell’Abruzzo e Molise “G. Caporale”, Teramo, Italy.
C. Batten, The Pirbright Institute, United Kingdom.
B. Hoffman, Friedrich-Loeffler-Institut, Germany.
S. Zientara, ANSES/INRA/ENVA, France.
-
Reviewed by
Project Management Board.
-
Date of submission by expert group
21 June 2019.
-
References
Agüero M, Gómez-Tejedor C, Angeles Cubillo M, Rubio C, Romero E, Jiménez-Clavero A. (2008) Real-time fluorogenic reverse transcription polymerase chain reaction assay for detection of African horse sickness virus. J Vet Diagn Invest. 20:325-8.
Alberca B, Bachanek-Bankowska K, Cabana M, Calvo-Pinilla E, Viaplana E, Frost L, Gubbins S, Urniza A, Mertens P, Castillo-Olivares J. (2014) Vaccination of horses with a recombinant modified vaccinia Ankara virus (MVA) expressing African horse sickness (AHS) virus major capsid protein VP2 provides complete clinical protection against challenge. Vaccine.;32:3670-4.
Bremer C.W., Huismans H. and Van Dijk A.A. (1990). Characterisation and cloning of the African horse sickness genome. Journal of General Virology. 72: 793-799.
Brown C.C., Meyer R.F. and Grubman M.J. (1994). Presence of African horse sickness virus in equine tissues, as determined by in situ hybridization. Veterinary Pathology. 31(6): 689-94.
Burrage T.G., Trevejo R., Stone-Marschat M. and W.W. L. (1993). Neutralising epitopes of African horse sickness virus serotype 4 are located on VP2. Virology. 196: 799-803.
Carpenter S., Mellor P.S., Fall A.G., Garros C., Venter G.J. (2017) African Horse Sickness Virus: History, Transmission, and Current Status. Annu. Rev. Entomol.. 62:343–58.
Carrasco L., Sanchez C., Gomez-Villamandos J.C., Laviada M.D., Bautista M.J., Martinez- Torrecuadrada J., Sanchez-Vizcaino J.M. and Sierra M.A. (1999). The role of pulmonary intravascular macrophages in the pathogenesis of African horse sickness. Journal of Comparative Pathology. 121(1): 25-38.
Chuma T, Le Blois H, Sánchez-Vizcaíno JM, Diaz-Laviada M, Roy P. (1992) Expression of the major core antigen VP7 of African horsesickness virus by a recombinant baculovirus and its use as a group-specific diagnostic reagent. J Gen Virol. 73:925-31.
Coetzer J.A.W. and Guthrie A.J. (2004). African Horse Sickness. In: Infectious Diseases of Livestock. J. A. W. Coetzer and R. C. Tustin. Oxford University Press. Southern Africa, 1231-1246.
Council Directive 2009/156/EC of 30 November 2009 on animal health conditions governing the movement and importation from third countries of equidae. Official Journal of the European Union
Durán-Ferrer M., Agüero M., Zientara S., Beck C., Lecollinet S., Saillou C., Smith S., Rueda P., Monaco F., Villalba R., Tena-Tomás C., Batten C., Gubbins S., Lubisi A., Sánchez-Vizcaíno JM., Emery M., Sturgil T., Ostlund E., Castillo-Olivares J. (2018). Assessment of Reproducibility of a VP7- BLOCKING ELISA diagnostic test for African horse sickness. Transbound Emerg Dis. 2019;66:83–90. doi: 10.1111/tbed.12968.
Du Toit, R.M. 1944. The transmission of bluetongue and horsesickness by Culicoides. Onderstepoort J. Vet. Res., 19:7-16.
European Food Safety Authority Efsa (2007). Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on request from the Commission on bluetongue vectors and vaccines. The EFSA Journal. (479): 1-29.
Gomez-Villamandos J.C., Sanchez C., Carrasco L., Laviada M.M., Bautista M.J., Martinez- Torrecuadrada J., Sanchez-Vizcaino J.M. and Sierra M.A. (1999). Pathogenesis of African horse sickness: ultrastructural study of the capillaries in experimental infection. Journal of Comparative Pathology. 121(2): 101-16.
Guthrie AJ, Quan M, Lourens CW, Audonnet JC, Minke JM, Yao J, He L, Nordgren R, Gardner IA, Maclachlan NJ. (2009) Protective immunization of horses with a recombinant canarypox virus vectored vaccine co-expressing genes encoding the outer capsid proteins of African horse sickness virus. Vaccine.;27:4434-8.
Hamblin C, Graham SD, Anderson EC, Crowther JR. (1990) A competitive ELISA for the detection of group-specific antibodies to African horse sickness virus. Epidemiol Infect. 104:303-12.
House C, Mikiciuk PE, Berninger ML. (1990) Laboratory diagnosis of African horse sickness: comparison of serological techniques and evaluation of storage methods of samples for virus isolation. J Vet Diagn Invest. 2:44-50.
Kanai Y, van Rijn PA, Maris-Veldhuis M, Kaname Y, Athmaram TN, Roy P. (2014) Immunogenicity of recombinant VP2 proteins of all nine serotypes of African horse sickness virus. Vaccine. 32:4932-7.
Lhafi A., Tber A., Fikri A. and Laghzaoui K. (1992). African horse sickness in Morocco: the epizootics of 1989 and 1990. In: Bluetongue, African horse sickness and related Orbiviruses. T. E. Walton and B. I. Osburn. CRC Press. Boca Raton, 205-216.
Laviada MD, Roy P, Sánchez-Vizcaíno JM and Casal I. (1995). The use of African horse sichness virus NS3 protein, expressed in bacteria, as a marker to differentiate infected from vaccinated horses. Virus Res., 38, 205–218.
Martinez-Torrecuadrada J.L., Diaz-Laviada M., Roy P., Sanchez C., Vela C., Sanchez-Vizcaino J.M. and Casal J.I. (1996). Full protection against African horsesickness (AHS) in horses induced by baculovirus-derived AHS virus serotype 4 VP2, VP5 and VP7. Journal of General Virology. 77(Pt 6): 1211-21.
Meiswinkel R. and Paweska J.T. (2003). Evidence for a new field Culicoides vector of African horse sickness in South Africa. Preventive Veterinary Medicine. 60(3): 243-53.
Mellor P.S. and Hamblin C. (2004). African horse sickness. Veterinary Research. 35(4): 445-66.
Mellor P.S., Boned J., Hamblin C., Graham S.D., (1990) Isolations of African horse sickness virus from vector insects made during the 1988 epizootic in Spain, Epidemiol. Infect. 105:447–454.
Portas M., Boinas F.S., Oliveira E.S.J., Rawlings P. and Oliveira E Sousa J. (1999). African horse sickness in Portugal: a successful eradication programme. Epidemiology & Infection. 123(2): 337-46.
Rodriguez M., Hooghuis H. and Castano M. (1992). African horse sickness in Spain. Veterinary Microbiology. 33(1-4): 129-42.
Sailleau C, Hamblin C, Paweska JT, Zientara S. (2000) Identification and differentiation of the nine African horse sickness virus serotypes by RT-PCR amplification of the serotype-specific genome segment 2. J Gen Virol. Mar;81(Pt 3):831-7.
Sellers RF, Pedgley DE, Tucker MR (1977). Possible spread of African horsesickness on the wind. J. Hyg. (Camb.), 79: 279-298.
Venter G.J., Graham S.D. and Hamblin C. (2000). African horse sickness epidemiology: vector competence of south african Culicoides species for virus serotypes 3, 5 and 8. Medical & Veterinary Entomology. 14(3): 245-50.
